Home / Product / Detail page
Qualitative test for COVID-19 total Ab
- U.S. FDA EUA approved
- 3 minutes | Fast result
- 2 steps | Easy to use
- 96.7% & 98.8% | Positive & Negative Percent Agreement
- Microfluidic Qualitative Immunoassay
- LIS connectivity (data management)
The FREND™ COVID-19 total Ab is a fluorescence immunoassay (FIA) which can be used to check whether patient has developed immune response to SARS-CoV-2 using human plasma. For COVID-19 total Ab, its detection is based on a fluorescent immunoassay showing qualitative result.
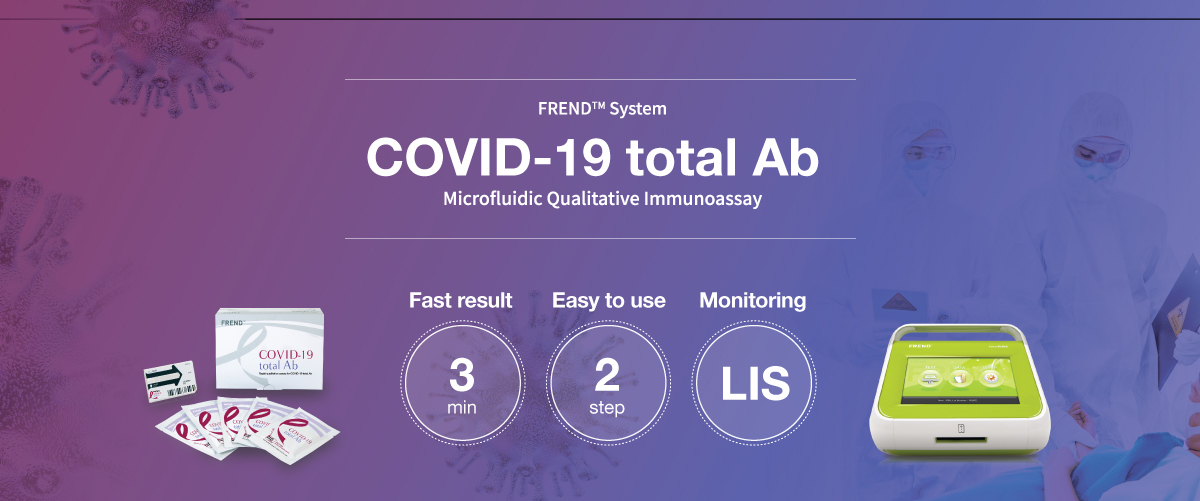
With just simple steps of operation, the FREND™ system supports quick decision-making.

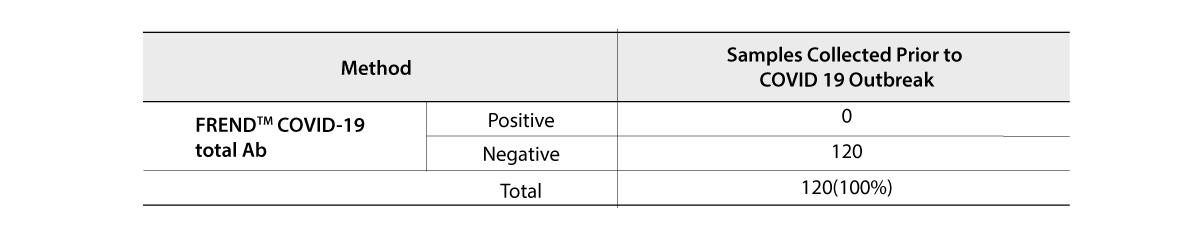
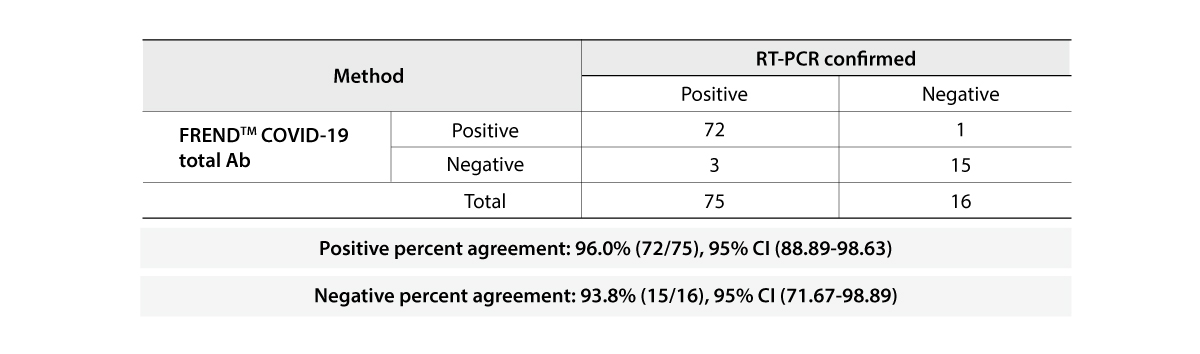
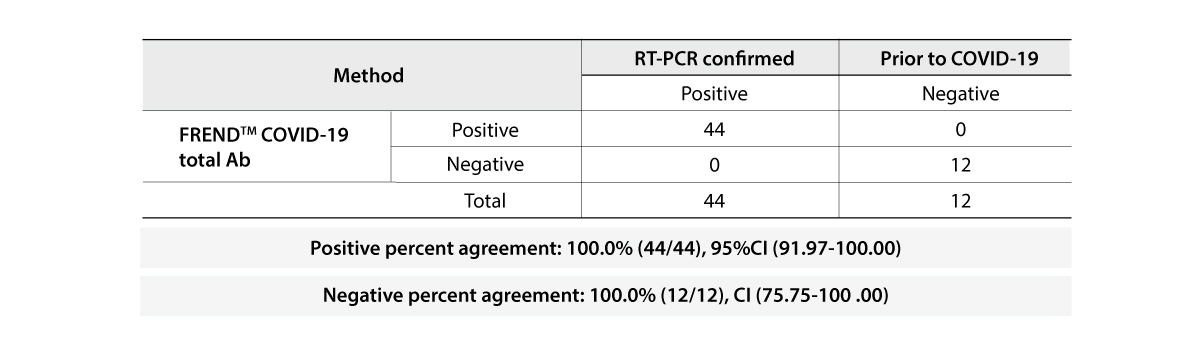
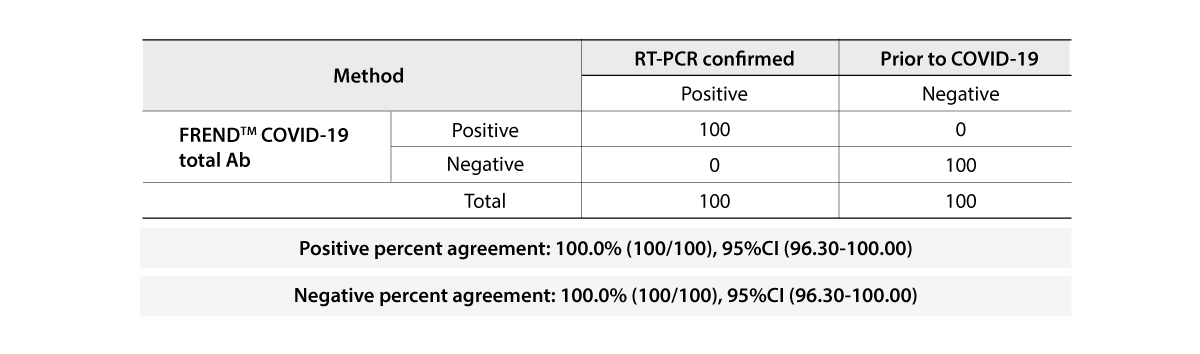
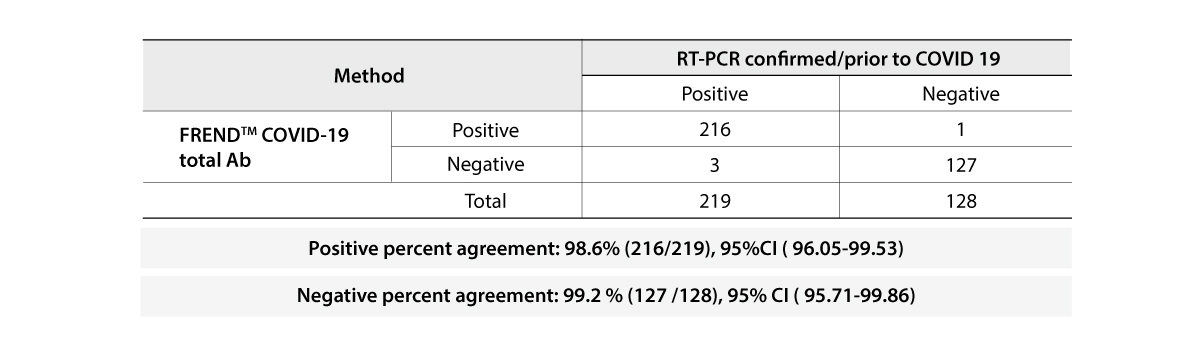
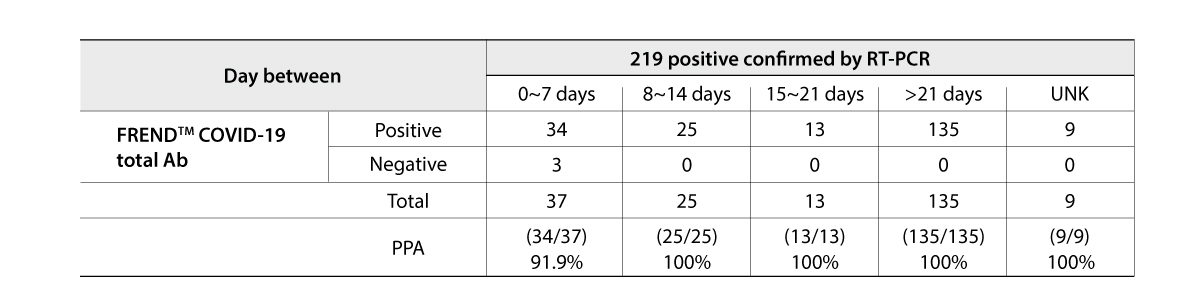
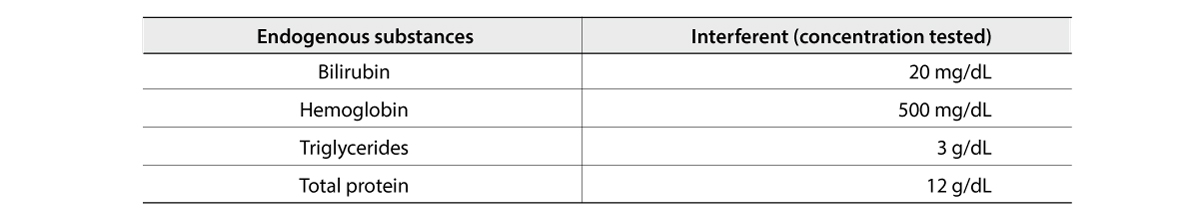
The FREND COVID-19 total Ab from NanoEntek was tested on August 19, 2020 at the Frederick National Laboratory for Cancer Research (FNLCR) sponsored by the National Cancer Institute (NCI).


Effective Data Management
-
Assay method
Fluorescent immunoassay
-
Cat. No.
FRCOA 020
-
Specimen
EDTA Plasma
-
Volume
35 μL
-
Time to result
Cartridge reaction : < 3 min
-
Package
20 tests
-
Storage condition
2–8 °C (35–46 °F)

ExTransfection™ Protocol Library
ExTransfection™ provides open protocols that make it easy for users to set up and run experiments. Its 24-well optimization method helps users to optimize transfection protocols easily and quickly.
ExTransfection™ also offers a pre-programmed optimization protocol that helps an easy and quick optimization of electrical parameters for both adherent and suspended cells.

 KOR
KOR ENG
ENG