Home / Product / Detail page
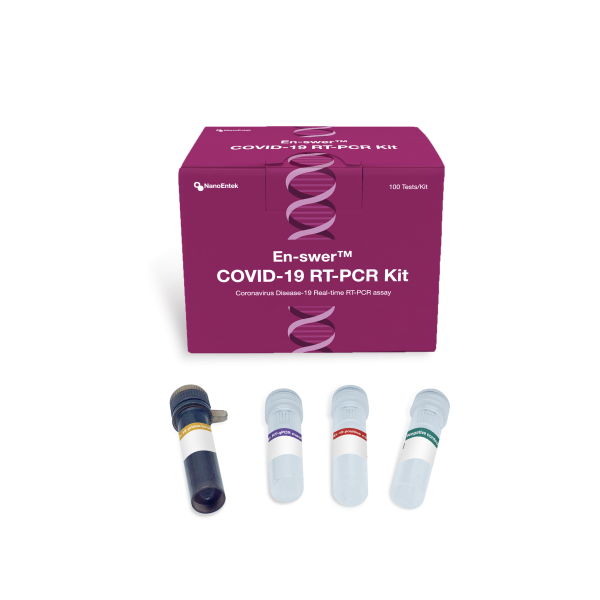
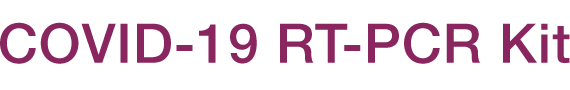
1-step Real-Time RT-PCR assay
- about 1 hour 30 min | Test time
- 1 tube reaction | Easy to use
- E gene, N gene (Target), RNaseP (IPC) | Detection
- High Sensitivity & Specificity
- Based on WHO & CDC recommended gene
Total Solution for COVID-19
1-step Real-Time RT-PCR assay
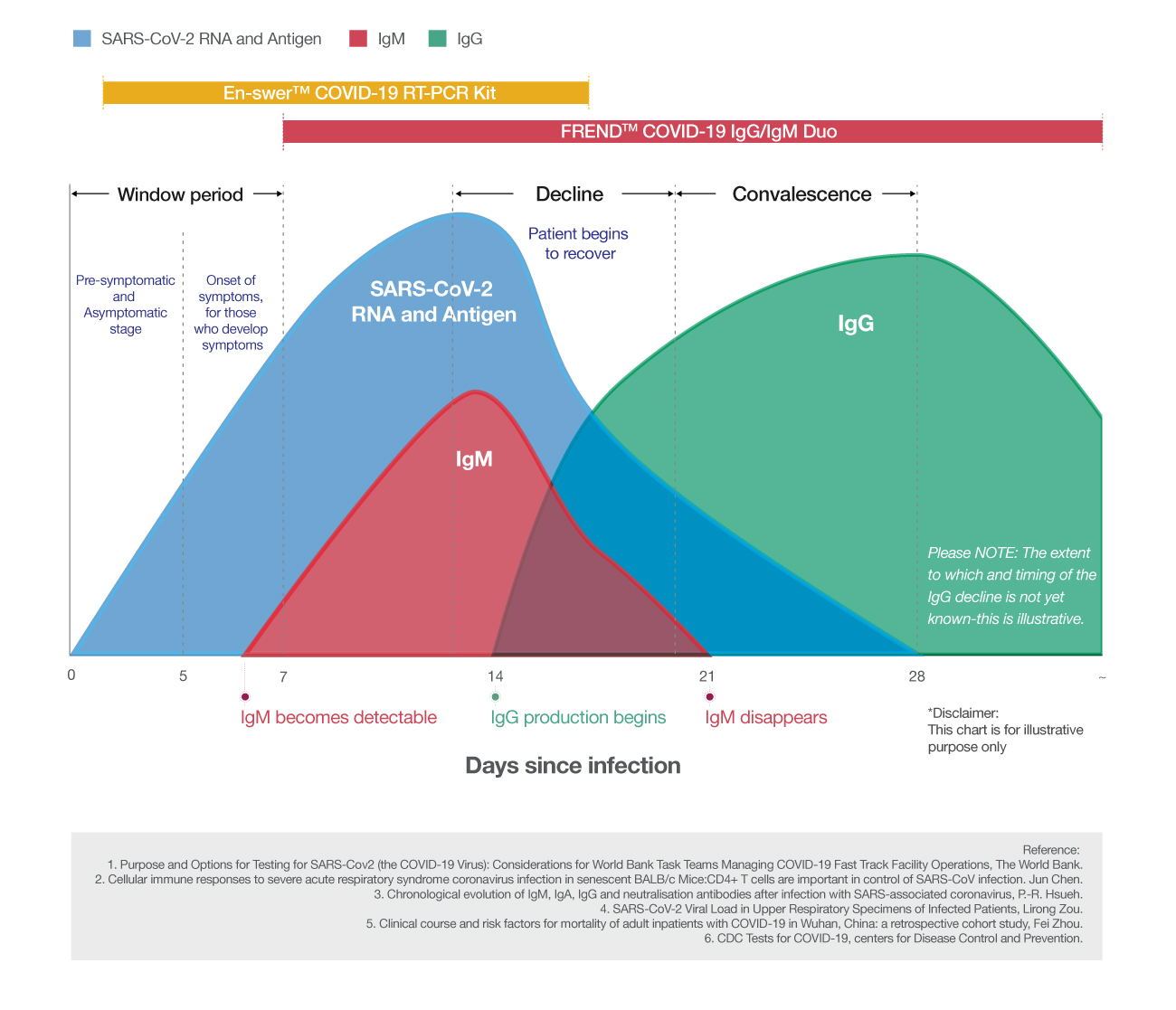
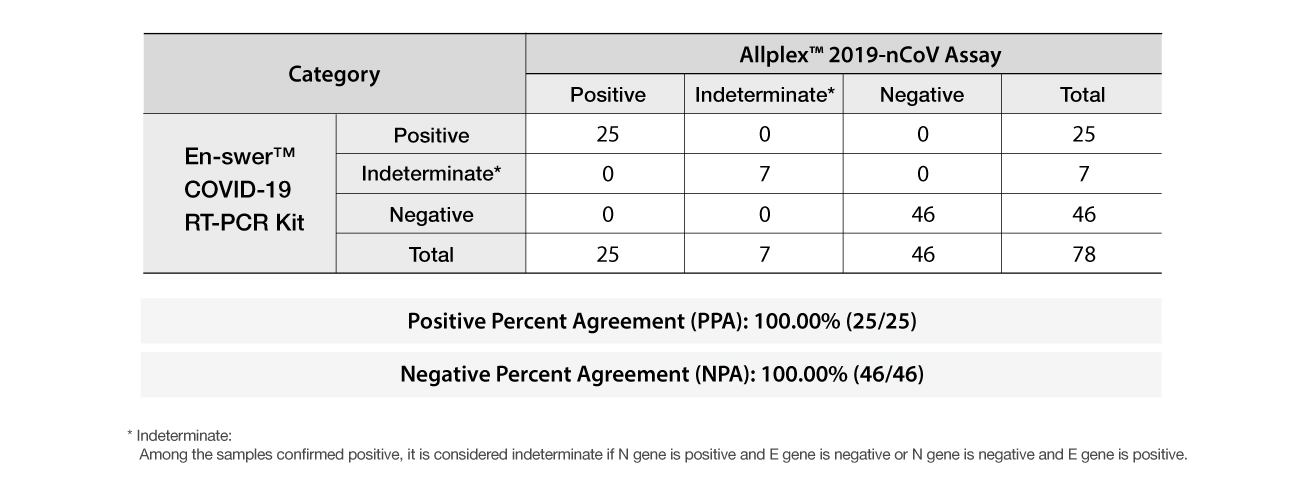
The En-swer™ COVID-19 RT-PCR Kit is an in-vitro diagnostic medical device using 1-step RT-qPCR(real-time reverse-transcription polymerase chain reaction) which is a test method that detects genes(the N gene, the E gene) of the novel coronavirus(COVID-19) through qualitative analysis from sputum, nasopharyngeal swab of patients suspected of being infected with the novel coronavirus(COVID-19).

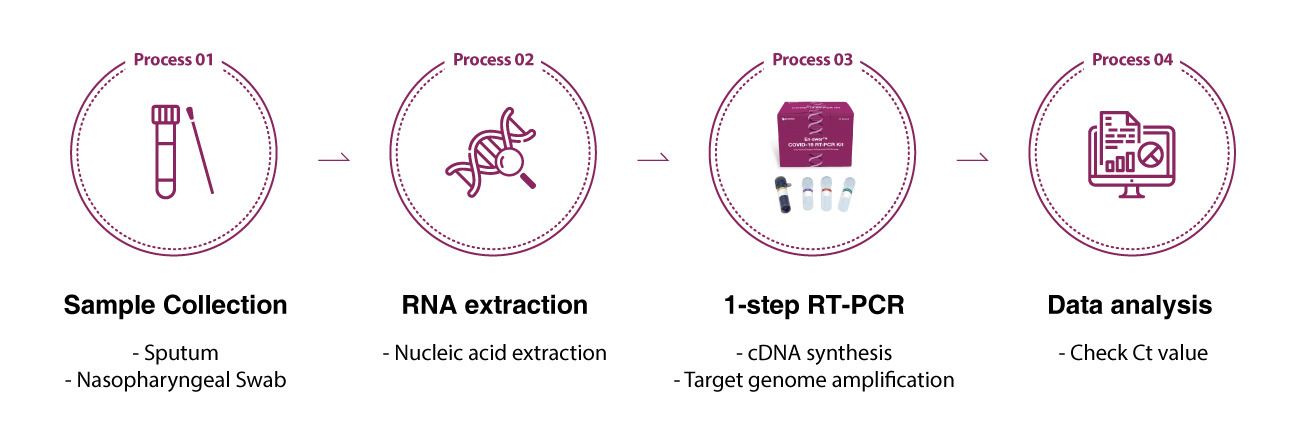
The following workflow is the instructions of using En-swer™ COVID-19 RT-PCR Kit.
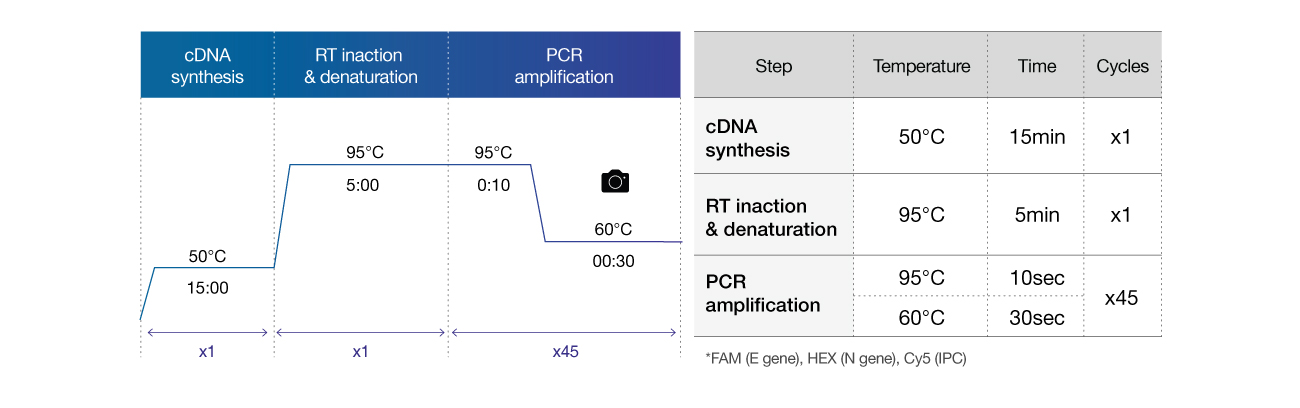
Cycle condition
The En-swer™ COVID-19 RT-PCR Kit can be used with Real-time PCR equipment including 7500 from Applied Biosystems and CFX96 from Bio-Rad. You can also use the equipment that can detect 3 different fluorescence such as FAM, HEX, Cy5, and so on. The response conditions are as follows.
Components
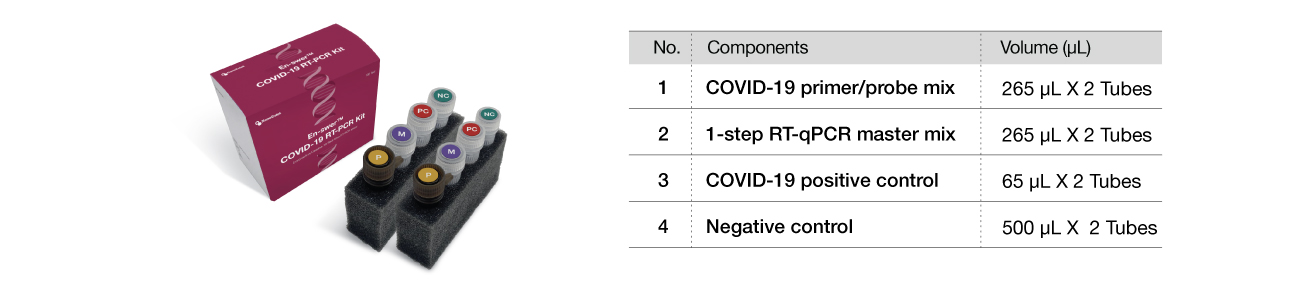
-
Cat. No.
EPCO19 100
-
Package
100 tests/Kit
-
Specimen
Sputum, Nasopharyngeal swab
-
Test time
–1 hour 30 min
-
Target gene
E gene, N gene
-
Storage
–20℃
-
Expiration date
12 months

ExTransfection™ Protocol Library
ExTransfection™ provides open protocols that make it easy for users to set up and run experiments. Its 24-well optimization method helps users to optimize transfection protocols easily and quickly.
ExTransfection™ also offers a pre-programmed optimization protocol that helps an easy and quick optimization of electrical parameters for both adherent and suspended cells.

 KOR
KOR ENG
ENG