Home / 응용분야 / 응용분야 전체

-

Cultured Meat: Sustainable Innovation & Role of High-throughput Automated Cell Counter
With advancements in biotechnology and a growing concern for the environmental impact of traditional animal farming, cultured meat has emerged as a promising solution that could revolutionize the way we produce and consume protein. Cultured meat is produced by culturing animal cells in a controlled environment, without the need for raising and slaughtering animals. NanoEntek’s high-throughput automated cell counters, EVE™ HT and EVE™ HT FL, can accelerate the production of cultured meat. NanoEntek’s high-throughput automated cell counters, EVE™ HT and EVE™ HT FL, can accelerate the production of cultured meat 1. What is Cultured Meat? Cultured meat, also known as lab-grown or cultivated meat, is produced by culturing animal cells to create meat products without the need for traditional livestock farming and slaughtering. This innovative approach involves isolating stem cells from animals such as cows or chickens and providing them with the necessary nutrients and environmental conditions to proliferate and differentiate into muscle, fat, and connective tissue cells. The resulting product closely resembles conventionally produced meat but offers significant advantages in terms of environmental sustainability, animal welfare, and potential health benefits. 2. Emerging Necessity of Cultured Meat (1) Continuous Growth of World’s population As of January 25, 2024, the global population has surpassed 8 billion and is projected to reach nearly 9 billion by 2050, according to real-time statistics from Worldometers. This population growth, coupled with shifting dietary habits towards increased meat consumption, especially in emerging economies, exacerbates ethical and environmental concerns associated with traditional meat production. (2) Growing demand for Meat Global meat production has surged from 70.57 million tons in 1961 to 337.18 million tons in 2020, driven by population growth and economic development. The Food and Agriculture Organization of the United Nations predicts that meat demand will double by 2050. Livestock farming is a major contributor to greenhouse gas emissions, accounting for 14.5% of global emissions, with cattle farming being the largest emitter due to methane release. 3. Benefit of Cultured Meat Cultured meat offers a sustainable solution to the environmental and ethical challenges posed by conventional meat production. With up to 92% lower greenhouse gas emissions, 90% less land use, 98% less soil acidification, and 94% less air pollution compared to conventional beef, cultured meat addresses key sustainability issues. Additionally, its production is not limited by geographical constraints, enhancing food security and production efficiency while reducing the risk of zoonotic diseases and antimicrobial resistance.4. How are cultured meat made? Cultivated meat production embodies the principles of biomimicry, a discipline that draws inspiration from natural processes to develop innovative technologies. In the context of cellular agriculture this entails replicating the inherent cellular growth mechanisms found in animals outside of their living organisms. The manufacturing process starts by isolating and banking stem cells from an animal, which can self-renew and differentiate. These cells are then cultivated in bioreactors, often referred to as cultivators, in large quantities. These cells are nourished with a nutrient-rich medium containing amino acids, glucose, vitamins, salts, and growth factors to stimulating the conditions inside the animal’s body.By adjusting the composition of the medium and providing cues from a scaffolding structure, the immature cells are prompted to develop into various tissues like skeletal muscle, fat, and connective tissues, which are the components of meat. Once the cells have differentiated, they are harvested, processed, and packaged into final products. This entire process typically takes between 2 to 8 weeks, depending on the type of meat being cultivated. 5. The Role of High-throughput Automated Cell Counter(1) NanoEntek’s EVE™ HT Series: Transforming Bioprocess Efficiency with Superior Throughput, Minimal Sample Requirements, and Swift Measurement Times The starting cell type ultimately influences many of the downstream variables of the bioprocess, including timelines and differentiation strategies. Accurate measurement of cell density during the initial seeding process is pivotal. A slight error in the seeding density can profoundly affect subsequent processes. Additionally, in bioreactors, it is necessary to culture a large number of cells rapidly. Therefore, periodic sampling and analyzing a substantial quantity of samples is essential in bioprocessing.From this perspective, NanoEntek’s high-throughput automated cell counters, EVE™ HT and EVE™ HT FL, have an edge over other conventional high-throughput cell counters in terms of throughput, required sample volume, and measurement speed.♦ Throughput Conventional high-throughput automated cell counters have the capacity of measuring only up to 24 samples maximum. However, EVE™ HT and EVE™ HT FL is capable of measuring up to 48 samples per each test, enhancing the productivity of the R&D and manufacturing process. Researchers and operator could as a result, easily and quickly find the optimal condition in extensive experiment with different combination of parameters. NanoEntek's EVE™ HT FL Multichannel counting plate: Up to 48 fluorescence-stained samples to be loaded at once.♦ Required Sample Volume Whereas conventional high-throughput cell counters demand up to 500 µL of sample per test, EVE™ HT and EVE™ HT FL stand out by requiring only a minimal 20µL per test. This significant reduction not only showcases EVE™ HT and EVE™ HT FL’s exceptional efficiency but also underscores their ability to conserve valuable samples ♦ Measurement Time For a batch of 48 samples, the EVE™ HT series completes the measurement process in only 3 minutes. In comparison, other equipment can take up to around 210 minutes. This represents a staggering time efficiency gap of nearly 70-fold, firmly establishing the EVE series as a leader in time efficiency.EVE™ HT FL is much more faster and requires substantially low sample volume compared to competitors.Given these compelling advantages, NanoEntek’s EVE™ HT and EVE™ HT FL high-throughput automated cell counters offer high efficiency and accuracy for the initial bioprocess stages.(2) The Integral Role of EVE™ HT and EVE™ HT FL in Cultured Meat ManufacturingNanoEntek’s high-throughput automated cell counters, the EVE™ HT and EVE™ HT FL, are indispensable tools in the cultured meat production process, especially during the initial manufacturing stages. These devices are pivotal in monitoring and analyzing cell cultures, ensuring their health and viability throughout the production cycle.By automating cell counting, these counters streamline operations, saving time and reducing labor while minimizing the risk of human error. Furthermore, they furnish researchers with precise and dependable data on cell growth and proliferation.High-throughput automated cell counters are essential for assessing the quality and consistency of cultured meat products, ensuring that each batch meets the desired specifications. Additionally, these counters play an essential role in process optimization, enabling researchers to detect and rectify potential issues at the onset of the production cycle.EVE™ HT and EVE™ HT FL measure up to 48 samples per test using only 20µL of sample, completing a batch measurement of 48 samples in just 3 minutes.NanoEntek's high-throughput automated cell counters, EVE™ HT and EVE™ HT FLConclusionIn conclusion, cultured meat has the potential to revolutionize the food industry by offering a sustainable, ethical, and healthy alternative to traditional meat. NanoEntek's high-throughput automated cell counters, EVE™ HT and EVE™ HT FL, are instrumental in realizing this potential by providing researchers with the tools they need to produce cultured meat products that meet the highest standards of quality and consistency.Overall, the development of cultured meat represents a significant step forward in addressing some of the most pressing challenges facing the food industry today. By harnessing the power of technology and innovation, we have the opportunity to create a more sustainable, ethical, and healthy food system for future generations.
-
EVE™ HT: Efficient, Accurate, and High-Throughput Solutions for Stem Cell analysis
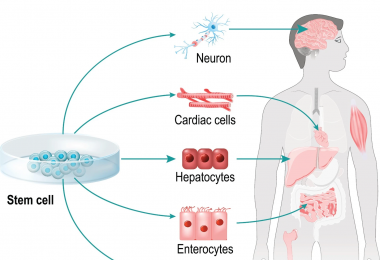
Summary Stem cell therapy involves using viable human stem cells to regenerate tissues and treat various diseases. It encompasses embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells. EVE™ HT uses trypan blue for quick and accurate viability evaluation in up to 48 samples within 3 minutes.Cell Therapy and its classificationCell therapy uses living cells to regenerate and restore damaged or diseased cells. It has applications in drug development, toxicology testing, and biomarker research. The three main types are tissue cell therapy, immune cell therapy, and stem cell therapy. The following content will primarily focus on stem cell therapy. The new treatment uses stem cell from various sources to regenerate cell or tissue types. What is Stem Cell therapy? Stem cell-based therapies are defined as any treatment for a disease or a medical condition that fundamentally involves the use of any type of viable human stem cells including embryonic stem cells (ESCs), iPSCs and adult stem cells for autologous and allogeneic therapies. Stem cells offer the perfect solution when there is a need for tissue and organ transplantation through their ability to differentiate into the specific cell types that are required for repair of diseased tissues. [1] Where Can I use Stem Cell therapy? Stem cells serve as the source for all cells, possessing undifferentiated characteristics, self-replication abilities, and the potential for differentiation. This differentiation function allows the treatment of damaged organs or tissues by promoting division and indefinite duplication. Stem cells are categorized into adult stem cells (AS cells), induced pluripotent stem cells (iPS cells), and embryonic stem cells (ES cells).Stem cell therapy, harnessing the capacity to differentiate and multiply into diverse cells, has been extensively studied and developed for various diseases, facilitated by technological advancements. Many developed countries offer policy support, recognizing it as a key component of the medical industry. Stem cells also serve as biomarkers to monitor post-treatment effectiveness and as cell treatments for incurable diseases.Through differentiation and proliferation, stem cells can be injected into patients to replace damaged cells, fostering tissue development in cell therapy. Additionally, stem cells play a role in enhancing the efficiency of new drug development by identifying candidates and assessing their efficacy. Stem cell therapy has shown effectiveness in treating a myriad of conditions, including burns, cardiovascular diseases, muscle diseases, vision impairment, and Alzheimer's disease. Additionally, stem cells hold promise for addressing challenging diseases such as spinal cord injuries, dementia, Crohn's disease, Parkinson's disease, Lou Gehrig's disease, and multiple sclerosis.Among various stem cell types, hematopoietic stem cells (HSCs) play a crucial role in producing red blood cells, white blood cells, platelets, and immune cells like T-lymphocytes and B-lymphocytes. Hematopoietic stem cell transplantation (HSCT) is a rapidly growing procedure worldwide, finding application in treating conditions such as aplastic anemia, acute leukemia, and solid cancers. EVE™ HT: Efficient, Accurate, and High-Throughput Solutions forWhen working with tissue-derived cells, particularly stem cells, accurate assessment of cell concentration and viability is essential for maintaining quality throughout subsequent procedures. A High Throughput Trypan Blue-Based Cell Counter, such as the EVE™ HT, is a valuable tool in the manufacturing process of stem cell therapy products, offering specific capabilities that contribute to the overall efficiency and quality control.EVE™ HT EVE™ HT is an automated cell counter that uses trypan blue for high-throughput measurements. In less than 3 minutes, it can accurately measure up to 48 samples, making it an efficient solution for busy laboratories or industries. The efficiency is maximized through the use of a disposable 48 channel plate.Here's a detailed explanation of how it can be utilized:Why is evaluating viability of cells important in Stem cell therapy?Therapeutic Efficacy: Ensuring PotencyThe therapeutic efficacy of stem cell therapies is directly linked to the potency of the administered cells. Only viable and functional stem cells have the potential to contribute to tissue regeneration or repair. Monitoring cell viability guarantees that the therapeutic product contains a sufficient number of viable cells capable of carrying out the intended therapeutic functions.Patient Safety: Minimizing RisksAdministering non-viable or compromised stem cells to patients can pose serious risks. Non-viable cells may not survive after transplantation, leading to treatment failure and potential harm to the patient. By assessing viability during the manufacturing process, the risk of administering ineffective or harmful cell products can be minimized.Consistency and Reproducibility: Quality ControlViability measurements contribute to maintaining consistent product quality. Quality control measures ensure that each batch of stem cell therapy meets predefined standards. Consistency in viability levels across batches enhances reproducibility, allowing for reliable therapeutic outcomes and facilitating regulatory compliance.Optimizing Dosing: Accurate Cell DosingDetermining the appropriate cell dose for each patient is crucial for the success of stem cell therapy. Viability data is essential for calculating the actual number of viable cells in a given dose, allowing clinicians to administer an optimal and standardized cell dose tailored to individual patient needs.Cost-Effectiveness: Efficient Resource UtilizationMonitoring cell viability helps avoid wasting resources on non-viable cells. Inefficient use of resources, such as culture media, supplements, and laboratory personnel time, can be minimized by eliminating non-viable cell populations early in the manufacturing process. This contributes to cost-effectiveness in the production of stem cell therapies.Post-Transplantation Outcomes: Predicting In Vivo PerformanceThe viability of stem cells before transplantation can influence their survival and behavior in the patient's body. Monitoring viability helps predict post-transplantation outcomes, providing valuable information for clinicians and researchers to optimize treatment protocols and improve long-term therapeutic benefits.Reference[1] Current state of stem cell-based therapies: an overview, Stem Cell Investigations
-
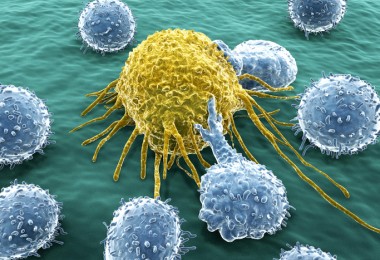
EVE™ HT FL: Elevating CAR-T Therapy through High Throughput Precision
Immune cell therapy, particularly CAR-T cell therapy, utilizes the body's immune system to combat diseases, primarily cancer. CAR-T cells are genetically modified T cells designed to target and destroy cancer cells. In the manufacturing process of CAR-T, high-throughput fluorescence Cell Counters like EVE™ HT FL play a crucial role. They assess cell viability, enumerate CAR-T cells, ensure quality control of starting material, optimize culture conditions, determine optimal harvesting time, detect contaminants, and facilitate data documentation for regulatory compliance, enhancing efficiency and quality in CAR-T manufacturing. In summary, EVE™ HT FL enhances efficiency and quality in CAR-T manufacturing, contributing to the success of this personalized immunotherapy. Cell Therapies: Tissue cell therapy, Immune cell therapy, and Stem cell therapy Cell therapies encompass tissue cell therapy, immune cell therapy, and stem cell therapy. In biomedicine, they stand as distinct fields, with cell therapy further divided into tissue-cell therapy, immune cell therapy, and stem-cell therapy. Gene therapy, on the other hand, is categorized into immune cell gene therapy and stem cell gene therapy. This discussion focuses on immune cell therapy, specifically CAR-T, and how high-throughput fluorescence Cell Counters such as EVE™ HT FL are applied in CAR-T manufacturing. Biomedicine Classification What is immune cell therapy?Immune cell therapy, also known as immunotherapy, is a medical approach that utilizes the body's immune system to combat diseases, particularly cancers and immune-related disorders. This personalized treatment involves extracting a patient's immune cells, genetically modifying them in the laboratory to enhance their targeting abilities, and reintroducing them into the patient's bloodstream. Its applications extend to new drug development, toxicology testing, and biomarker research. Notable examples include CAR-T cell therapy, where engineered T cells specifically target and destroy cancer cells. Immune cell therapy offers precision medicine tailored to an individual's immune profile and has shown remarkable success in certain cancers, opening new possibilities for treating previously challenging conditions. Ongoing research aims to expand its applicability and optimize safety and efficacy. What is CAR-T cell? CAR-T cells are T cells that undergo genetic modification to create an artificial T-cell receptor. This innovative immunotherapy aims to enhance the ability of T cells to identify and eliminate cancer cells. The process involves extracting T cells from individuals, altering them genetically, and then reintroducing the engineered CAR-T cells into patients to target and destroy tumors more effectively. These CAR-T cells can either come from the patient's own blood (autologous) or from a healthy donor's T cells (allogenic). To ensure safety, CAR-T cells are designed to specifically recognize antigens present on tumors but not on healthy cells. EVE™ HT FL: Efficient, Accurate, and High-Throughput Solutions for CAR-T productsWhen working with cells like PBMCs or T-cells, accurate assessment of cell concentration and viability is essential for maintaining quality throughout subsequent procedures. Cell counting in these kinds of samples becomes a challenging task due to common contaminants like red blood cells and non-cellular debris, introducing complexities such as time consumption, subjectivity, and a higher likelihood of errors.EVE™ HT FL When working with cells like PBMCs or T-cells, accurate assessment of cell concentration and viability is essential for maintaining quality throughout subsequent procedures. Cell counting in these kinds of samples becomes a challenging task due to common contaminants like red blood cells and non-cellular debris, introducing complexities such as time consumption, subjectivity, and a higher likelihood of errors.The EVE™ HT FL is a high-throughput automated fluorescence cell counter equipped with bright field and two fluorescence channels (AO/DAPI).AO (Acridine Orange) and DAPI (4′, 6-diamidino-2-phenylindole) are fluorescent dyes that bind to DNA, staining the nucleus of targeted cells. AO is a permeable dye that stains the nucleus of all cells, regardless of the cell’s condition. DAPI is an impermeable dye that only stains the nucleus of non-viable cells with damaged membranes. Basic principle of counting - Single reagent of EVE™ HT FL To accurately evaluate the viability of cell types like PBMCs or stem cells, it is advisable to use dual-fluorescence staining. This method effectively excludes non-nucleated cells (e.g., RBCs), platelets, and debris from the samples. In just 3 minutes, up to 48 samples can be counted and analyzed. How can EVE™ HT FL be applied in the manufacturing process of CAR-T?When working with cells like PBMCs or T-cells, accurate assessment of cell concentration and viability is essential for maintaining quality throughout subsequent procedures. Cell counting in these kinds of samples becomes a challenging task due to common contaminants like red blood cells and non-cell.1. Cell Viability Assessment:EVE™ HT FL, a high throughput fluorescence Cell Counter enable quick and accurate determination of the percentage of viable CAR-T cells, in other words, real-time monitoring of cell viability. Moreover, it allows for real-time monitoring of cell growth and proliferation. In CAR-T manufacturing, where a large number of T cells are often needed, this capability is crucial for for ensuring the overall quality of the final CAR-T product. By tracking these parameters, researchers can optimize culture conditions to ensure the production of a sufficient number of viable CAR-T cells.2. Enumeration of CAR-T Cells:EVE™ HT FL streamlines the enumeration process of CAR-T cells. By automating cell counting, it excel in enumerating total cell counts, including both viable and non-viable cells, further facilitating the determination of cell concentration. This allows for accurate seeding of cells during various stages of manufacturing process. This is crucial for maintaining the desired cell density and optimizing downstream processes.3. Quality Control of Starting Material:The success of CAR-T therapy is highly dependent on the quality of the starting T cell population. EVE™ HT FL facilitate a comprehensive evaluation of cellular morphology, size, and overall quality. Ensuring that the starting T cells meet predetermined criteria is essential for consistent and effective CAR-T production.4. Optimization of Culture Conditions:Continuous monitoring of cell cultures provides insights into the effects of different culture conditions on CAR-T cell growth and health. Researchers can use this information to optimize media composition, incubation parameters, and other variables, leading to enhanced CAR-T cell production efficiency.5. Determination of Optimal Harvesting Time:EVE™ HT FL aid in determining the optimal harvesting time for CAR-T cells. By monitoring cell viability during the culture period, researchers can identify the point at which the cells exhibit maximum viability and are most suitable for harvesting, optimizing the overall manufacturing timeline.6. Detection of Contaminants or Abnormalities:AO/DAPI staining can highlight contaminants or abnormalities within the cell population. Deviations in cell morphology or the presence of unwanted cell types can be quickly identified, allowing for corrective actions to be taken to maintain the purity of the CAR-T cell product.ConclusionIn conclusion, EVE™ HT FL can be applied in CAR-T manufacturing, offering capabilities for assessing cell viability, enumerating cell counts, monitoring cell growth, and ensuring the overall quality of the CAR-T cell product. Its high-throughput nature contributes to the efficiency and success of the manufacturing process, ultimately impacting the therapeutic efficacy of CAR-T cell therapies.
-
Revolutionizing Cell Counting: NanoEntek's Evolution from Hemocytometer to Cutting-edge Innovations
Pioneering a New Era in Cell Counting Technology Introduction: Cell counting has been a cornerstone in scientific and medical research, providing critical insights into biological processes and cell viability. The journey began with Louis-Charles Malassez's invention of the hemocytometer, a groundbreaking device that laid the foundation for cell counting. However, as technology advanced, NanoEntek emerged as a key player, revolutionizing the process and simplifying cell counting through a series of innovative products. The Origin of Cell Counting and Development: Hemocytometer Louis-Charles Malassez's hemocytometer marked the inception of cell counting, offering a precise method for counting blood cells. The Neubauer counting chamber became a standard tool in laboratories. This traditional method, while effective, presented inconveniences that inspired NanoEntek to pioneer advancements.Hemocytometer Counting Grid NanoEntek’s Continuous innovation towards Cell counting1) NanoEntek’s innovation in Simplifying Cell Counting: C-chip In response to the inconvenience associated with traditional glass slide hemocytometer, NanoEntek invented ‘C-chip’ - a disposable plastic hemocytometer. The C-chip eliminated the need for washing and cover slips, streamlining the cell counting process while maintaining accuracy. This innovation was a pivotal step in simplifying and modernizing cell counting techniques. 2) NanoEntek’s Innovation Continues: EVE™, EVE™ Plus NanoEntek introduced faster and more accurate automated cell counters, namely 'EVE™' and 'EVE™ Plus.' These devices not only expedited the counting process but also incorporated automated image analysis, enhancing the assessment of cell viability. The presentation of data in graphical formats further improved user convenience, allowing for more comprehensive and insightful analysis. NanoEntek’s Product Line-up for Cell Counting1) Automated Cell counter NanoEntek's product lineup for cell counting includes the C-chip (2ch/4ch), a disposable hemocytometer known for its high accuracy and time-saving features. EVE™ Plus stands out as one of the fastest cell counters, capable of counting cells within 1 second. For precise cell counting, ADAM™ MC2 utilizes PI staining technology. Notably, ADAM™ CellT meets the regulatory standards of 21CFR Part 11, making it suitable for use in cGMP production environments. NanoEntek's automated cell counters 1) Automated Cell counter NanoEntek's product lineup for cell counting includes the C-chip (2ch/4ch), a disposable hemocytometer known for its high accuracy and time-saving features. EVE™ Plus stands out as one of the fastest cell counters, capable of counting cells within 1 second. For precise cell counting, ADAM™ MC2 utilizes PI staining technology. Notably, ADAM™ CellT meets the regulatory standards of 21CFR Part 11, making it suitable for use in cGMP production environments. 2) High-throughput automated cell counter(1) EVE™ HTEVE™ HT is an automated cell counter that uses trypan blue for high-throughput measurements.In less than 3 minutes, it can accurately measure up to 48 samples, making it an efficient solution for busy laboratories or industries. The efficiency is maximized through the use of a disposable 48 channel plate. For those in regulated environments, EVE™ HT offers an optional 21CFR Part11 compliance, ensuring data integrity and security.EVE™ HT(2) EVE™ HT FLEVE™ HT FL is a high-throughput dual fluorescence cell counter.Equipped with a bright field and two fluorescence channels, namely AO and DAPI, EVE™ HT FL exceeds in speed and accuracy. In fact, its accuracy is evidenced with the R squared value of 0.99 it shows when compared to a flow cytometer. EVE™ HT FL can accurately measure up to 48 samples in less than 3 minutes, making it an efficient solution for busy laboratories or industries. The efficiency is maximized through the use of a disposable 48-channel plate.Remarkably, EVE™ HT FL only requires 20µL of sample per each measurement, which allows users to substantially reduce sample consumption by up to 25 times less than the competing instruments. This not only promotes efficient usage of the samples, but also let users to preserve their precious samples for additional evaluations or experiments.EVE™ HT FL offers an outstanding high-throughput cell counting method suitable for research and development in bio-processing and cell therapies. Its combination of speed, accuracy, and sample efficiency makes it a valuable tool in various scientific applications.EVE™ HT FLNanoEntek’s InnovationFor the past 20 years, NanoEntek has consistently led in innovation, introducing creative and groundbreaking products. Our dedication to sharing inventive ideas for the continuous development of our world is unwavering. Through a relentless pursuit of creativity, we aim to make meaningful strides in enhancing our global community.
-

ADAMⅡ™-CD3/CD4/CD8
ADAMⅡ-CD3/CD4/CD8 ADAMII-CD3/CD4/CD8 Kit is compatible with ADAMII. It is used for counting CD3+, CD3+CD4+, and CD3+CD8+ cell that can be used in research laboratory. General Description ADAMII–CD3/4/8 is a bench-top fluorescence cell counter that counts CD3, CD4, and CD8 expressing cells. ADAMII–CD3/4/8 is an image-based cytometer and it takes up to 75 sets of images from sample loaded in a disposable ADAMII Assay Slide. Each set of images comprises one bright-field image and three fluorescence images. These images are processed to identify CD3 expressing T-lymphocytes, CD4 expressing helper T-lymphocytes, and CD8 expressing cytotoxic T-lymphocytes. Principle ADAMII T-cell assay starts with adding an appropriate volume of a sample to a single test tube and mixing the sample with reagent solution containing fluorescence-labeled antibodies. Once the staining step is completed, RBC Lysis Solution is added to lyse red blood cells in the sample. The prepared sample is loaded into a disposable ADAMII Assay Slide and the slide is inserted into ADAMII instrument. ADAMII –CD3/4/8 reports absolute counts of CD3+ T-cells (cells/μL), (2) absolute counts of CD3+/CD4+ T-helper cells (cells/μL), (3) absolute counts of CD3+/CD8+ T-killer cells (cells/μL), (4) ratio of T-helper cells over T-killer cells. Simple and Easy of use Flow cytometry is the standard method for enumerating T lymphocyte cells it has some limitations, such as expensive instrumentation and reagent costs, and poor reproducibility between technicians and laboratories. ADAMII is easy to use. Sample preparation steps are shown in the cartoon on the right-hand side. ADAMII doesn’t require routine cleaning and maintenance. Pumping, cleansing and waste bottles are not necessary for the ADAMII.Results The ADAMII simultaneously captures a series of bright field and fluorescent images of the sample in the assay slide and runs a comprehensive image analysis on the images to determine cell counts. With the well-defined geometry of ADAMII Assay Slide, ADAMII reports not only the ratio but also the absolute counts of T cells. Sample images are shown below. A) Bright: Total cells are counted. Shapes and sizes of objects are used to eliminate non-cell debris. B) CD3 channel: combining with BF channel, CD3+ cells are counted. C) CD8 channel: combining with CD3 channel, CD3+/CD8+ cells are counted. D) CD4 channel: combining with CD3 channel, CD3+/CD4+ cells are counted. Accuracy Total 30 whole blood samples were used to compare ADAMII and flow cytometry. Linear regression analysis revealed a close correlation between the data for the absolute number of human helper/inducer, suppressor/cytotoxic, and total T lymphocytes obtained with the ADAMII and flow cytometry (Absolute number of CD3+, R2 = 0.998; Absolute number of CD3+/CD4+, R2 = 0.993; Absolute number of CD3+/CD8+, R2=0.992). Detailed data are shown in the graphs below. Precision (within-run) Total T lymphocytes (CD3+), helper/inducer(CD3+CD4+), suppressor/cytotoxic(CD3+CD8+) of precision test was conducted using whole blood with 6 different concentrations. Each concentration was tested in 6 biological replicates and 3 technical replicates per preparation. The coefficient of variation of total T lymphocytes (CD3+) and T lymphocytes subsets (CD3+/CD4+, and CD3+/CD8+) were below 10%. Detailed data are shown in the tables below. Operator to operator Three operators performed CD3/CD4/CD8 count at low concentration and showed low CV values which demonstrated high accuracy in operator to the operator comparison test. Instrument to instrument Three instruments performed CD3/CD4/CD8 count at low concentration and showed low CV values which demonstrated high accuracy in instrument to the instrument comparison test. Specifications
-

COVID-19 Total Solution
NanoEntek provides a total solution from diagnosis to vaccine development and patient monitoring of COVID-19. En-swer™ COVID-19 RT-PCR Kit detects the presence of SARS-CoV-2 virus itself through real-time reverse-transcription polymerase chain reaction. FREND™ COVID-19 IgG/IgM Duo can be used to check whether patients have developed immune responses to SARS-Cov-2 using human plasma. ADAMII™-CD3/CD4/CD8 counts the T-cells to monitor the immune status of human immunodeficiency that can be used for vaccine development and monitoring for the COVID-19 patients.Is RT-PCR enough? Is serology test enough? Now add T-cell ! Common Usage of T-Cell Count Counting the T cell subsets accurately and rapidly is important. The T-cell count has already been used to monitor patients and its importance is increasing in the COVID-19 era. Originally, the T cell count has been used to monitor T-cell responses in immunocompromised patients like AIDS or patients suffering from immunodeficiency (1). For example, CD4 T lymphocytes count is used to determine or consider the need of antiretroviral therapy (ART) for AIDS patients (2). In addition, frequent monitoring of CD4+ T cells is essential to overall monitor the disease progression as well as immune suppression (3). Allogeneic stem cell transplantation is performed to restore blood cells in cancer patients who have gone through chemotherapy. However, graft-versus-host disease mediated by T cells are major complication causing fatal consequences after allogeneic stem cell transplantation. Several researches have suggested that the high CD3+ graft content was associated with the increased risk of either acute or chronic graft-versus-host disease without any beneficial effect on the disease control (4,5). These studies suggest that the assessment of CD3+graft content may aid in reducing severe GVHD risk without having the negative impact on transplantation outcomes.Why T- cell count in the post-COVID-19 era? Understanding how COVID-19 affects our body is essential to fight COVID-19. Researchers are putting effort to reveal how COVID-19 can cause severe respiratory symptoms in human. Zhang et al. has shown that the prominent feature of COVID-19 was progressive lymphocytopenia and notably CD3 T cells were affected the most as well as CD4 and CD8 T cells (6). There were also two different studies which monitored the patients in different conditions using the immunological parameters. When it comes to the ICU patients, the severity of COVID-19 showed inverse relationship with T-cell count (7). However, another study showed that the high number of CD4 and CD8 T cells were found in the COVID-19 patients at the convalescent stages (8). Such studies have highlighted the importance of counting T cells in the COVID-19 era and further researches are required to elucidate the relationship between COVID-19 and CD4 and 8 T cells. Monitoring T-cell is essential in Vaccine development The development of COVID-19 vaccine has become inevitable. As vaccine aims to elicit immune responses through CD4 T cells, understanding the dynamics of T-cell is essential in the vaccine development. Currently, more than 152 vaccines are under the development and the spike protein is the major antigen of interest (9). Actual studies were performed to show whether the spike protein elicits specific CD4 T cells or CD8 T cells responses. Grifoni et al. proved that spike protein can specifically elicit specific CD4 T cells which show high correlation with the IgG responses (8). Feng-Cai Zhu et al. published the result of first-in-human trial using adenovirus type-5 vectored COVID-19 vaccine by testing through dose-escalation and monitored up to 28 days (10). It was found that INF-γ+CD4 T and CD8 T cells increased after 14 days.Then why ADAMII™-CD3/CD4/CD8? The ADAMII™-CD3/CD4/CD8 identify the absolute counts of human T lymphocytes(CD3+), helper/inducer(CD3+CD4+) T lymphocytes, and suppressor/cytotoxic(CD3+CD8+) T lymphocytes in RBC-lysed whole blood. It targets of T cell responses to SARS-CoV-2 in human, so it can be used for vaccine development and monitoring for the COVID-19 patients.Save TIME, save MONEY. It's easy to use! Our ADAMII™-CD3/CD4/CD8 provides absolute T cell counts within 17 minutes and help you to save your time and money. It will support you to better understand about the COVID-19.It provides accurate absolute number of CD3+/CD4+/CD8+ cells with rapid turnaround and minimal hands-on time for the operator. The high degree of precision of the system is due in part to the simplifying sample preparation steps, elimination of washing and reconstituting steps which may cause cell loss and human error. Reference (1) World Health Organization (2017). Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. https://www.who.int/hiv/pub/arv/arv-2016/en/ (2) World Health Organization, Regional Office for South-East Asia. (2009). Laboratory guidelines for enumeration CD4 T lymphocytes in the context of HIV/AIDS (revised version 2009). WHO Regional Office for South-East Asia. https://apps.who.int/iris/handle/10665/205403 (3) Ramalingam, V.V., Mani, M., Sundaresan, V.C., Karunaiya, R.J., Sachithanandham, J. and Kannangai, R. (2012). Daily Quality Control in CD3+and CD4+T Cell Estimation by the FACS Count System at a Tertiary Care Center in South India. Clinical and Vaccine Immunology, 19(10), pp.1693–1696. (4) Paz Morante, M., Briones, J., Canto, E., Sabzevari, H., Martino, R., Sierra, J., Rodriguez-Sanchez, J.L. and Vidal, S. (2006). Activation-associated phenotype of CD3+ T cells in acute graft-versus-host disease. Clinical and Experimental Immunology, 145(1), pp.36–43. (5) Czerw, T., Labopin, M., Schmid, C., Cornelissen, J.J., Chevallier, P., Blaise, D., Kuball, J., Vigouroux, S., Garban, F., Lioure, B., Fegueux, N., Clement, L., Sandstedt, A., Maertens, J., Guillerm, G., Bordessoule, D., Mohty, M. and Nagler, A. (2016). High CD3+ and CD34+ peripheral blood stem cell grafts content is associated with increased risk of graft-versus-host disease without beneficial effect on disease control after reduced-intensity conditioning allogeneic transplantation from matched unrelated donors for acute myeloid leukemia — an analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Oncotarget, 7(19), pp.27255–27266. (6) Zhang, X., Tan, Y., Ling, Y., Lu, G., Liu, F., Yi, Z., Jia, X., Wu, M., Shi, B., Xu, S., Chen, J., Wang, W., Chen, B., Jiang, L., Yu, S., Lu, J., Wang, J., Xu, M., Yuan, Z., Zhang, Q., Zhang, X., Zhao, G., Wang, S., Chen, S. and Lu, H. (2020). Viral and host factors related to the clinical outcome of COVID-19. Nature. (7) Diao, B., Wang, C., Tan, Y., Chen, X., Liu, Y., Ning, L., Chen, L., Li, M., Liu, Y., Wang, G., Yuan, Z., Feng, Z., Zhang, Y., Wu, Y. and Chen, Y. (2020). Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Frontiers in Immunology, [online] 11. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7205903/pdf/fimmu-11-00827.pdf [Accessed 21 Jun. 2020]. (8) Grifoni, A., Weiskopf, D., Ramirez, S.I., Mateus, J., Dan, J.M., Moderbacher, C.R., Rawlings, S.A., Sutherland, A., Premkumar, L., Jadi, R.S., Marrama, D., de Silva, A.M., Frazier, A., Carlin, A., Greenbaum, J.A., Peters, B., Krammer, F., Smith, D.M., Crotty, S. and Sette, A. (2020). Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. [online] Available at: https://www.cell.com/cell/pdf/S0092-8674(20)30610-3.pdf [Accessed 22 May 2020]. (9) COVID-19 Vaccine Tracker. (n.d.). COVID-19 Vaccine Tracker. [online] Available at: https://biorender.com/covid-vaccine-tracker. (10) Zhu, F.-C., Li, Y.-H., Guan, X.-H., Hou, L.-H., Wang, W.-J., Li, J.-X., Wu, S.-P., Wang, B.-S., Wang, Z., Wang, L., Jia, S.-Y., Jiang, H.-D., Wang, L., Jiang, T., Hu, Y., Gou, J.-B., Xu, S.-B., Xu, J.-J., Wang, X.-W., Wang, W. and Chen, W. (2020). Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet, [online] 0(0). Available at:https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31208-3/fulltext.
-
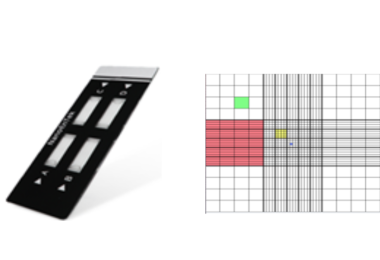
Cell counting development
The origin of cell counting and developmentThe hemocytometer (or haemocytometer) is counting-chamber device originally designed and usually used for counting blood cells. [1]The hemocytometer was invented by Lousi-Charles Malassez and consists of a thick glass microscope slide with a rectangular indentation that creates a chamber. This chamber is engraved with a laser-etched grid of perpendicular lines. The device is carefully crafted so that the area bounded by the lines and the depth of the chamber are known. By observing a defined area of the grid. It is therefore possible to count the number of cells or particles in a specific volume of fluid, and thereby calculate the concentration of cells in the fluid overall. A common type of hemocytometer is the Neubauer counting chamber. [2]How NanoEntek has developed to make cell counting simple and easyAs the cell counting has become necessary more and more, NanoEntek has helped to reduce time and effort of cell counting. NanoEntek invented an automated cell counter which has been sold more than 10,000 devices. By this, the global marketplace of automated cell counting has been launched. To reduce inconvenience of hemocytomer, C-chip has been invented by NanoEntek. C-chip has no need to wash because it is disposable. C-chip does not require coverslips. It reduces time and effort but have a high accuracy. After releasing C-chip, NanoEntek started to develop automated cell counter. NanoEntek also released faster and more accurate automated cell counters 'EVE' and 'EVE PLUS'. As the cell counters are made, it became possible to count the cells and check viability with automated image analysis.The data can be seen as a graph which makes more convenient for users. NanoEntek's products for cell countingNanoEntek has released various products for purpose of cell counting. C-chip (2ch / 4ch) is a disposable hemocytometer that reduces time and effort but has a high accuracy. EVE Plus is one of the fastest cell counters that counts cells in one minute. ADAM-MC2 is an accurate cell counter by using PI staining. ADAM-CellT complies with 21 CFR part 11 which is available in cGMP production environment. NanoEntek's innovationNanoEntek has developed by inventing creative and innovative products over 20 years. NanoEntek will continue to innovate and share our creative thoughts to develop our world more and more. Reference [1] Absher, M. (1973). Hemocytometer counting. In Tissue culture (pp. 395-397). Academic Press. Link: https://doi.org/10.1016/B978-0-12-427150-0.50098-X[2] Quinlan, L. R. (2006). Phosphoinositides, inositol phosphates, and phospholipase C in embryonic stem cells. In Embryonic Stem Cell Protocols (pp. 127-149). Humana Press., Link: https://doi.org/10.1385/1-59745-037-5:127
-
Stem cell therapy
A useful CD34 counter for stem cell therapy, ADAMII-CD34Stem cell therapy, which is seen as a cure for various diseases, is expected to be worth about 3.38 billion USD in 2023. As the demand increase for cell therapies and research actively progress, the accurate total cell number and viability have become the main factors as well as reproducibility.NanoEntek's ADAMII-CD34 provides reasonable device price and inspection costs for stem cell transplantation and treatment compared to competing equipment. There are advantages to using simple training without any experts, and it reduces the maintenance cost since the kits are disposable"What is the Stem cell therapy?" Cell therapy is the drug that induces regeneration for restoring damaged or diseased cells by using the living cell. It can be used for new drug development, toxicology testing, bio-marker and so on. Cell therapies can be divided into three parts; tissue cell therapy, immune cell therapy and stem cell therapy. We will specifically focus on the stem cell therapy. Biomedicine can be divided into cell therapy and gene therapy, and cell therapy can be subdivided into tissue-cell therapy, immuno-cell therapy and stem-cell therapy. Also, gene therapy can be subdivided into immune cell gene therapy and stem cell gene therapy [1]. Treatment through embryonic stem cellsMacula, where most of the visual cells are concentrated, is in charge of an important role in vision. Macular degeneration is a disease that degrades the function of the macular and causes blindness. The patient who have macular degeneration and Stargardt disease with no cure got to hear good news. Roski eye institute from the University of Southern California transplanted retinal pigment epithelium cells (RPE cells) from embryonic stem cells into four patients with macular degeneration. As a result, all of them have cured from the disease, sme of them have improved their eyesight. [2]In addition, clinical results were announced by May 2015 that improved vision through embryonic stem cell therapy. Where can I use the stem cell therapy? The stem cell is the source of all cells. It is a cell that is undifferentiated, and it can be self-replicating and be differentiated. The damage organs or tissues can be treated using the differentiation function because they can be divided and duplicated indefinitely. Stem cells can be classified into an adult stem cells (AS Cell), induced pluripotent stem cells(iPS cells) and embryonic stem cells (ES cells).Stem cell therapy that have the ability to differentiate and multiply into various cells. Many studies have been conducted and manufactured to apply stem cell therapy to various diseases according to technological advances. In addition, the majority of developed countries provide policy support as an one of the medical industries. The stem cell is used as a criterion by biomarker to monitor the effectiveness of post-treatments, and it can be used as cell treatments for incurable diseases.Stem cells can be injected into patients through differentiation and proliferation. It can replace damaged cells, and tissue functions can be developed as cell therapy. In addition, the stem cell can contribute to the efficiency of new drug development by finding new drug candidates and examining their efficacy. Stem cells, which have shown effects such as treating burn patients, cardiovascular diseases, treating muscle disease, recovering eye sight, and Alzheimer;s disease, are also expected to treat diseases such as, spinal cord injury, dementia, Crohn's disease, Parkinson's disease, Lou Gehrig's disease, and multiple sclerosis.Among stem cells , Hematopoietic stem cell (HSC) produces red blood cells (RBC, Erythocyte), white blood cells (WBC, Leukocyte), platelet and immune cell such as T-lymphocytes, B-lymphocytes. The hematopoietic stem cell is used to treat aplastic anemia, acute leukemia, and solid cancer. Hematopoietic stem cell transplantation (HSCT) is rapidly increasing worldwide. Why choose NanoEntek?Comparison ADAMII-CD34 with flow cytometry The traditional method used for cell counting is called flow cytometry represented by a fluorescence-activated cell sorter.The flow cytometry is expensive, and it is also inconvenient due to the size of equipment and incidental factors. Additional work for management before and after using the equipment also takes a lot of reagents, time and effort. When applying a strong electric field to cell separation, there are disadvantages of high damage to the cell and the need of an expert in the operation and management of the equipment. Features of ADAMII-CD34In what ways ADAMII-CD34 can be usedThe ADAMII-CD34 is a fluorescent cell counter through four optics (bright, yellow, red, green), which provides quick and convenient confirmation of Total CD45, Viable CD45, Viability of CD45, Total CD34, Viable CD34, Viability of CD34, Viable CD34 of Viable CD45, which are the main variables for determining stem cell extraction, storage and transplantation within up to 15 minutes. (Variable in different modes: 6 to 15 minutes). This device can be used in cell therapy center or clinics, laboratory or central lab in university, institute and hospital, blood bank and cord blood bank.A convenient way of counting stem cellsThe ADAMII-CD34 provies an accurate and precise results with rapid turnaround and minimal hands-on time by the operator. The high degree of precision of the system is due in part to the simplifying sample preparation steps and eliminating washing and reconstituting steps, which may cause cell loss and human error. In addition, results are reported using the test specific software, limiting interpretation steps by the user.The stem cell therapy is carried out through adult stem cells, cord blood and clinical trials. A lot of research is going all around the world by medical professionals. The range of treats that stem cells is able to treat will be expanding more.Therefore, NanoEntek has provided a variety of stem cell automated counters to support the development and productization of various stem cell. Research and development in stem cell will continue to expand and increase, so they can help to provide various equipment for quality control in stem cell therapyReference paper of using ADAM-CellT[1] Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies, Cell, 183 (1), pp. 126-142.e17, October, 01, 2020. Link: https://doi.org/10.1016/j.cell.2020.08.022[2] Expression of the transcription factor PU.1 induces the generation of microglia-like cells in human cortical organoids, Nature Communications, 13, 430, 2022 Link: https://doi.org/10.1038/s41467-022-28043-y[3] Heterozygous frameshift variants in HNRNPA2B1 cause early-onset oculopharyngeal muscular dystrophy, Nature Communications, 13, 2306, 2022 Link: https://doi.org/10.1038/s41467-022-30015-1Reference paper of using ADAM II-CD34[1] Enumeration of CD34 positive Stem Cells Using the ADAMII Image-based Fluorescence Cell Counter, Annals of Laboratory Medicine, 39(4), 388-395. 2019. Link: https://doi.org/10.3343/alm.2019.39.4.388[2] Clinical Applicability of Newly Developed Image-based Cell Counter for Counting CD34 + Cells: Comparison with Flow Cytometric Analysis, Clinical Pediatric Hematology-Oncology, 23, pp. 125-132,2016 Link: http://doi.org/10.15264/cpho.2016.23.2.125Reference[1] Future of Cell Therapy in the Regenerative Medicine Market (2016), Frost&Sullivan[2] Kashani Amr H., et al. "A bioengneered retinal pigment epthelial monolayer for advanced, dry age-related macular degeneration."Science translational medicine 10,435 (2018): eaao4097
-
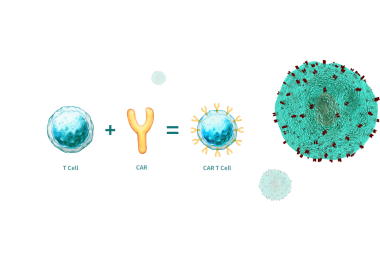
Immune cell therapy
All-in-one system of Immune therapy, ADAM-MC2, ADAM-CellT, and ADAM-CDxIn 2017, the FDA approved CAR-T cells (Chimeric antigen receiver T-cell) drug that had an excellent effect on blood cancer. As demand for CAR-T increases, research has been significantly developed. A high accuracy and reproducibility have become important as the total immune cell number and viability have become major variables. NanoEntek's ADAM-MC2, and ADAM-CellT can be used in the CAR-T manufacturing process. In addition,. ADAMⅡ-CDx** is all-in-one equipment that enables the CAR-T process to provide reasonable device prices and inspection costs compared to competing equipment. There is an advantage of using the equipment without any training or experts, and it reduces the maintenance cost since the kits are disposable.**ADAM-CDx from NanoEnTek is currently under development. This device can be used as an all-in-one in the cell therapy’s manufacturing process and R&D from leukapheresis to product QC "What is the Immune cell therapy?" Cell therapy is the drug that induces regeneration for restoring damaged or diseased cells by using the living cell. It can be used for new drug development, toxicology testing, bio-marker and so on. Cell therapies can be divided into three parts; tissue cell therapy, immune cell therapy and stem cell therapy. We will specifically focus on the latest issue which is Immune cell therapy. Biomedicine can be divided into cell therapy and gene therapy, and cell therapy can be subdivided into tissue-cell therapy, immuno-cell therapy and stem-cell therapy. Also, gene therapy can be subdivided into immune cell gene therapy and stem cell gene therapy [1].We will learn more about immuno-cell therapy in detail by using the CAR-T(Chimeric antigen receptor T-cell), which shows excellent treatment efficiency in recent blood cancer. CAR-T cells definition and their applications Cell therapy (also called cellular therapy or cytotherapy) is therapy in which cellular material is injected, grafted or implanted into a patient; this generally means intact, living cells. For example, T cells are capable of fighting cancer cells via cell-mediated immunity may be injected in the course of immunotherapy.Chimeric antigen receptor T cells (also known as CAR-T cells) are T cells that have been genetically engineered to produce an artificial T-cell receptor. The premise of CAR-T immunotherapy is to modify T cells to recognize cancer cells in order to more effectively target and destroy them. Scientists harvest T cells from people, genetically alter them, and then infuse the resulting CAR-T cells into patients to attack their tumors. CAR-T cells can be either derived from T cells in a patient's own blood (autologous) or derived from the T cells of another healthy donor (allogenic). For safety, CAR-T cells are engineered to be specific to an antigen expressed on a tumor that is not expressed on healthy cells.Figure 2. origin of CAR-T cell Types of CD marker and their functionsImmunocytotherapy is a treatment that takes immune cells, which are the core of cancer resistance, cultivates, activates and then transplants them back to attack cancer. One of the immuno-cell treatments, the CAR-T (Chimeric antigen receptor expression T-cell), is called "Living Drug" because it features the ability to kill tumor cells using its own T-cell, which continues to grow and live after infusion. [2]Groups of cell surface antigen or clusters of differentiation are typically written as the CD. A CD is a name given to identity and study cell surface molecules according to their immune expressions.[3]For the success of immuno-cell therapy transplants, the number of positive cells in major differentiation cluster (CD) such as mature T-cells (CD3), helper T-cells (CD4), killer T-cells or suppressor T-cells (CD8) is an important factor.Why choose NanoEntek?ADAM-MC series Cell Therapeutic Applications After ADAM-MC series can be used as a device for monitoring and QC of the cell numbers and viability in the process of manufacturing cells (CAR-T cells, stem cells, etc.) for cell therapy. In addition, it is possible to use ADAM-MC2 depending on the cell types (Whole blood cell, PBMCs, etc.) that needs to be monitored during the monitored during the manufacturing of cell therapy products. Figure 3. ADAM-MC2 applications More than 100 cell therapy research institutes and companies around the world use ADAM-MC and ADAM-MC2.Figure 4. collaborative institutes and companies with our products Comparison ADAM series with flow cytometryAs the global demand for cell therapies, research and production are increasing, and the accurate number of total cell counting and viability have become a main factor as well as reproducibility. The traditional method used for cell number and analysis is a flow cytometer represented by a FACS (fluorescence-activated cell sorter) [4]Figure 5. comparison our product with FACS However, the flow cytometer is expensive, and it is also inconvenient due to the size of equipment and incidental factors. Additional work for management before and after using the equipment also takes a lot of reagents, time and effort. When applying a strong electric field to cell separation, there are disadvantages of high damage to the cell and the need for an expert in the operation and management of the equipment.NanoEntek CAR-T's PlatformCAR-T manufacturing process Figure 6. NanoEntek CAR-T's platform. NanoEntek provides various tools like ADAM-MC2 and ADAM-CellT. They can be utilized in the manufacturing process from leukapheresis to product QC.It is easy to monitor all different steps of the purification, expansion, and formulation of CAR-T cells using the ADAM-MC series to ensure precise and reliable results. ADAM-MC series can be used for R&D, process control and quality control of CAR-T cell.After extracting the patient;s blood, the CAR-T cell manufacturing process isolates the T-cell from the blood through the leukapheresis and activates the T-cell through Anti-CD3 / Anti-CD28 Abcoated beads.Afterward, CAR-T is created after CAR gene expression using the gene transfer, and the CAR gene is transduced into activated T-cell. Before infusing it back into the patient, the number of CAR-T cells is increased through culture step, harvests and refrigerates them. [5] Our New Innovative product, ADAMⅡ-CDx (coming soon) Figure 7. application of ADAMⅡ-CDx ADAM-CDx from NanoEntek is currently under development. This device can be used as an all-in-one in the cell therapy's manufacturing process and R&D from leukapheresis to product QC.ADAM-CDx is specialized for various cells such as the T cell, B cell and NK cell to identify types of cell and utilized various functions such as total cell counting, cell size and cell cycling.Reference paper of using ADAM-MC2 [1] Hybrid SMART spheroids to enhance stem cell therapy for CNS injuries. Science advances, 7(40), eabj2281. Link: https://doi.org/10.1126/sciadv.abj2281[2] Plasma complement C7 as a target in non-small cell lung cancer patients to implement 3P medicine strategies. EPMA Journal, 629-645. https://doi.org/10.1007/s13167-021-00266-x[3] Adoptive immunotherapy with transient anti-CD4 treatment enhances anti-tumor response by increasing IL-18Rαhi CD8+ T cell, Nature Communications, 12(1), 1-15, 2021 Link: https://doi.org/10.1038/s41467-021-25559-7[4] IL7-Fc Enhances the Efficacy of Adoptive T Cell Therapy under Lymphopenic Conditions in a Murine Melanoma Model. Cells 2021, 10, 2018. Link: https://doi.org/10.3390/cells10082018[5] TDP-43 and PINK1 mediate CHCHD10 S59L mutation–induced defects in Drosophila and in vitro. Nature Communications, March, 2021. Link: https://doi.org/10.1038/s41467-021-22145-9[6] Polymorphic Region-Specific Antibody for Evaluation of Affinity-Associated Profile of Chimeric Antigen Receptor, Molecular Therapy Oncolytics, 17, pp. 293-305, 2020. Link: https://www.sciencedirect.com/science/article/pii/S2372770520300541[7] Chronic activation of 4-1BB signaling induces granuloma development in tumor-draining lymph nodes that is detrimental to subsequent CD8+ T cell responses, Cellular & Molecular Immunology, August 2020. Link: https://doi.org/10.1038/s41423-020-00533-3Reference paper of using ADAM-CellT [1] Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies, Cell, 183 (1), pp. 126-142.e17, October, 01, 2020. Link: https://doi.org/10.1016/j.cell.2020.08.022[2] Expression of the transcription factor PU.1 induces the generation of microglia-like cells in human cortical organoids, Nature Communications, 13, 430, 2022 Link: https://doi.org/10.1038/s41467-022-28043-y[3] Heterozygous frameshift variants in HNRNPA2B1 cause early-onset oculopharyngeal muscular dystrophy, Nature Communications, 13, 2306, 2022 Link: https://doi.org/10.1038/s41467-022-30015-1Reference[1] Future of Cell Therapy in the Regenerative Medicine Market(2016), Frost&Sullivan, 나노엔텍 재가공[2] Viardot, Andreas, et al. "Chimeric antigen receptor(CAR) T-cell therapy as a treatment option for patients with B-cell lymphomas: perspectives on the therapeutic potential of Axicabtagene ciloleucel." Cancer management and research 11(2019): 2393[3] CHAN, J. K. C., NG, C. S., HUI, P. K.(1988). “A simple guide to the terminology and application of leucocyte monoclonal antibodies”. 《Histopathology》 12(5): 461–480.[4] Huh, D.; WeiGu; Kamotani, Y.; Grotberg, J. B.; Takayama, S.Physiologicla Measurement 2005, 26, R73-R98.[5] 채희정, and 윤덕현. "광범위큰 B 세포림프종에 대한 키메릭항원수용체 T-세포(Chimeric Antigen Receptor T-cells) 치료." Korean Journal of Medicine(구 대한내과학회지) 94.2(2019): 152-158.

 KOR
KOR ENG
ENG