Home / Product / Detail page


Automated Cell Counter
- Automated image analysis
- Accurate result
- Sensitive CCD detection and precise automatic stage
- Auto focusing
A New Standard of Automated Cell counter
- Less than 1 min to get results

New Standard of Automated Cell Counting
The ADAM™ series automatic cell counter measures total cell numbers and cell viability through cutting-edge detection technologies.
Instead of trypan blue staining which can lead to inaccurate data, ADAM™ utilizes sensitive fluorescence dye staining, LED optics and CCD detection technologies.
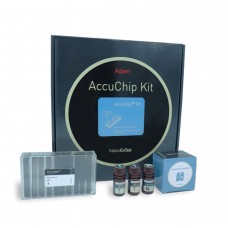
Disposable Microchips
The ADAM™ series automated cell counter utilizes a precision disposable microchip to eliminate the problem of a permanent counting chamber.
Each microchip is designed for single use. Pumping, cleansing, and wasting slides are not necessary for the ADAM™ cell counter.
Each microchip is produced at our state-of-the-art manufacturing facility and validated for accuracy.
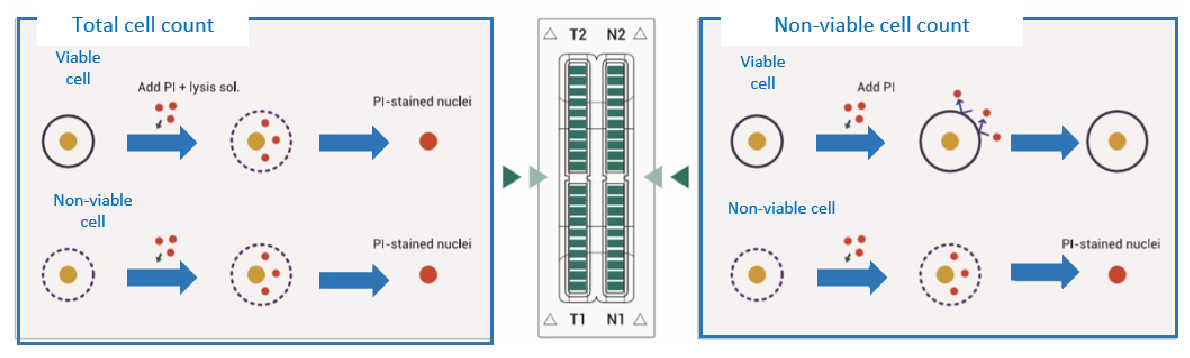
Accuracy & Reproducibility
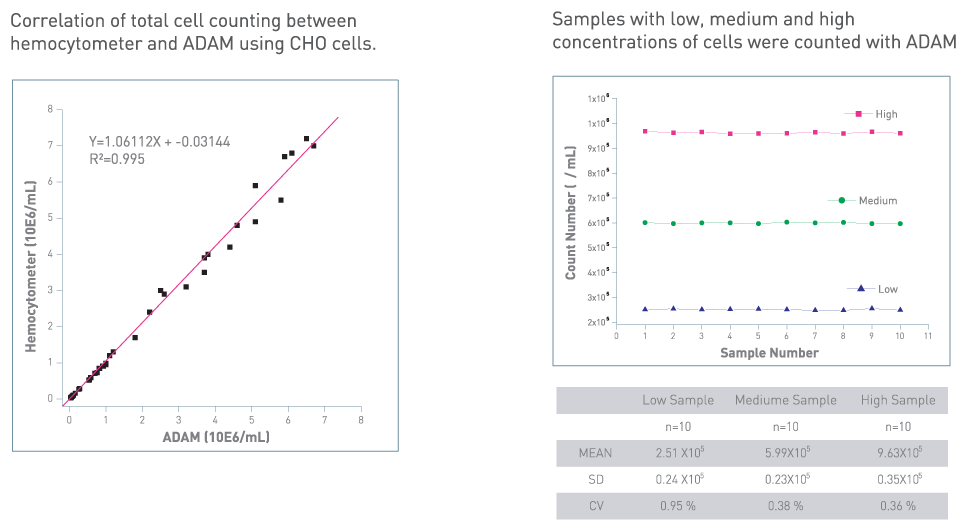
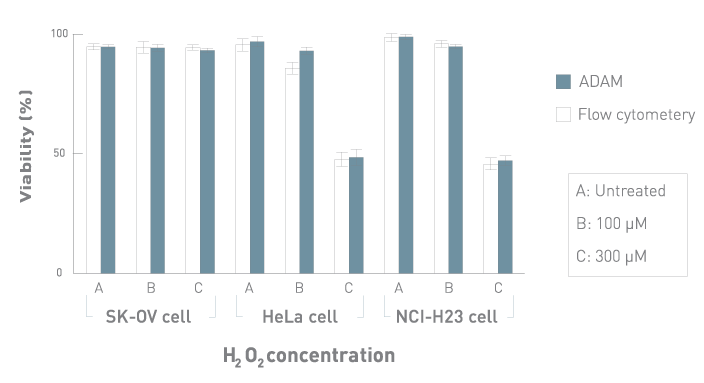
Comparison with Flow Cytometry
Comparison of cell viability between ADAM™ and flow cytometry.
SK-OV, HeLa and NCI-H23 cells were treated with 100 and 300 μM of H2O2 for 3 hours, then analyzed by ADAM™ and flow cytometry.
Values are given as mean ± SD of three experiments.

NanoEntek blog is now open!
Want to learn more about cell counting and cell therapy?
Visit to find out more!
-
Product name / Cat. No.
ADAM–MC / ADAM–MC
-
Loading Volume
13 μL / test (AccuChip 4ch)
-
Measuring Volume
3.1 μL / test (AccuChip 4ch)
-
Measure range
5 x 10E4–4 x 10E6 cell / mL
-
Focus
Auto focusing
-
LED
4W Green LED
-
Weight
9.0 kg
-
Size
220 (W) x 375 (L) x 250 (H) mm
-
Analysis time
< 1 min (AccuChip 4ch)
-
Dyes
PI (Propidium Iodide)
-
Connectivity
N/A
-
Cell type
Cell line (clumpy cell, single cell), PBMCs, Adipose Stem cell, Total white blood cell, Primary cells

ExTransfection™ Protocol Library
ExTransfection™ provides open protocols that make it easy for users to set up and run experiments. Its 24-well optimization method helps users to optimize transfection protocols easily and quickly.
ExTransfection™ also offers a pre-programmed optimization protocol that helps an easy and quick optimization of electrical parameters for both adherent and suspended cells.

 KOR
KOR ENG
ENG