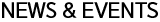Home / Application / Detail page

Immune cell therapy
All-in-one system of Immune therapy, ADAM-MC2, ADAM-CellT, and ADAM-CDx
In 2017, the FDA approved CAR-T cells (Chimeric antigen receiver T-cell) drug that had an excellent effect on blood cancer. As demand for CAR-T increases, research has been significantly developed. A high accuracy and reproducibility have become important as the total immune cell number and viability have become major variables.
NanoEntek's ADAM-MC2, and ADAM-CellT can be used in the CAR-T manufacturing process. In addition,. ADAMⅡ-CDx** is all-in-one equipment that enables the CAR-T process to provide reasonable device prices and inspection costs compared to competing equipment. There is an advantage of using the equipment without any training or experts, and it reduces the maintenance cost since the kits are disposable.
**ADAM-CDx from NanoEnTek is currently under development. This device can be used as an all-in-one in the cell therapy’s manufacturing process and R&D from leukapheresis to product QC
"What is the Immune cell therapy?"
Cell therapy is the drug that induces regeneration for restoring damaged or diseased cells by using the living cell. It can be used for new drug development, toxicology testing, bio-marker and so on. Cell therapies can be divided into three parts; tissue cell therapy, immune cell therapy and stem cell therapy. We will specifically focus on the latest issue which is Immune cell therapy.
Biomedicine can be divided into cell therapy and gene therapy, and cell therapy can be subdivided into tissue-cell therapy, immuno-cell therapy and stem-cell therapy. Also, gene therapy can be subdivided into immune cell gene therapy and stem cell gene therapy [1].
We will learn more about immuno-cell therapy in detail by using the CAR-T(Chimeric antigen receptor T-cell), which shows excellent treatment efficiency in recent blood cancer.

Types of CD marker and their functions
Immunocytotherapy is a treatment that takes immune cells, which are the core of cancer resistance, cultivates, activates and then transplants them back to attack cancer. One of the immuno-cell treatments, the CAR-T (Chimeric antigen receptor expression T-cell), is called "Living Drug" because it features the ability to kill tumor cells using its own T-cell, which continues to grow and live after infusion. [2]
Groups of cell surface antigen or clusters of differentiation are typically written as the CD. A CD is a name given to identity and study cell surface molecules according to their immune expressions.[3]
For the success of immuno-cell therapy transplants, the number of positive cells in major differentiation cluster (CD) such as mature T-cells (CD3), helper T-cells (CD4), killer T-cells or suppressor T-cells (CD8) is an important factor.
Why choose NanoEntek?
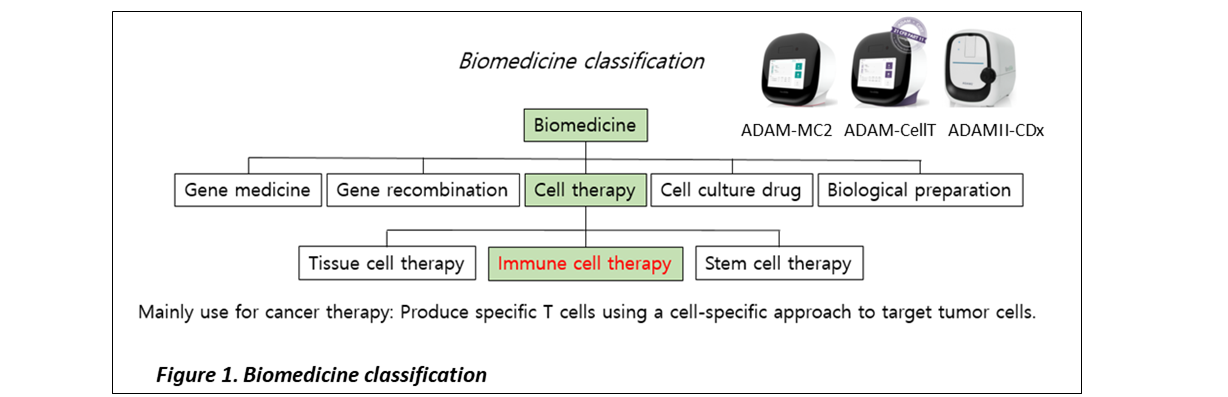
ADAM-MC series Cell Therapeutic Applications
After ADAM-MC series can be used as a device for monitoring and QC of the cell numbers and viability in the process of manufacturing cells (CAR-T cells, stem cells, etc.) for cell therapy. In addition, it is possible to use ADAM-MC2 depending on the cell types (Whole blood cell, PBMCs, etc.) that needs to be monitored during the monitored during the manufacturing of cell therapy products.
More than 100 cell therapy research institutes and companies around the world use ADAM-MC and ADAM-MC2.

Figure 4. collaborative institutes and companies with our products
NanoEntek CAR-T's Platform
CAR-T manufacturing process
Figure 6. NanoEntek CAR-T's platform. NanoEntek provides various tools like ADAM-MC2 and ADAM-CellT. They can be utilized in the manufacturing process from leukapheresis to product QC.
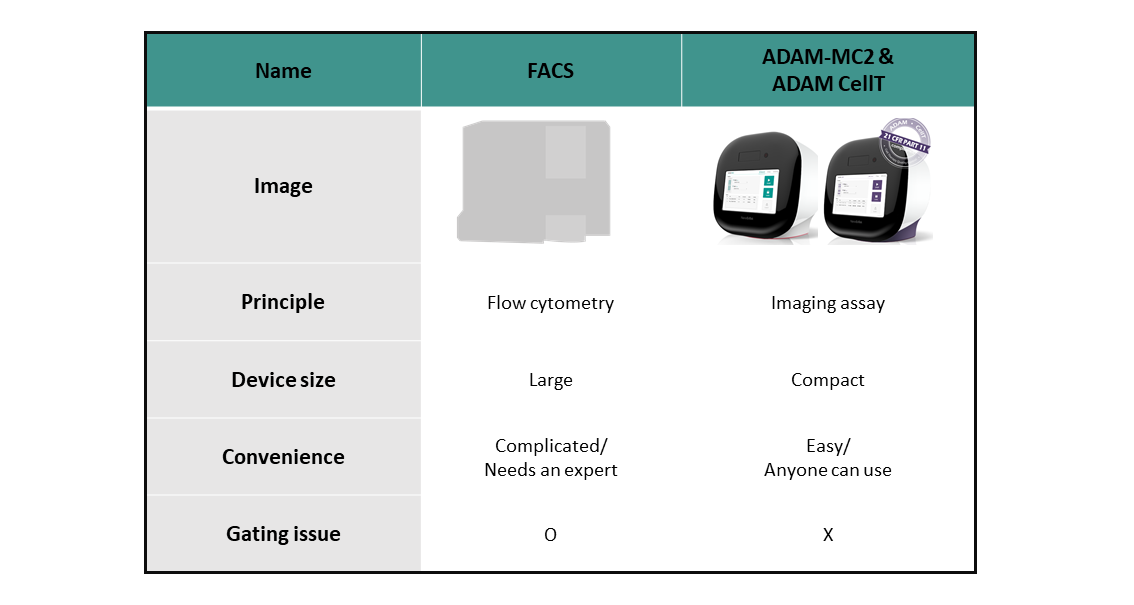
It is easy to monitor all different steps of the purification, expansion, and formulation of CAR-T cells using the ADAM-MC series to ensure precise and reliable results. ADAM-MC series can be used for R&D, process control and quality control of CAR-T cell.
After extracting the patient;s blood, the CAR-T cell manufacturing process isolates the T-cell from the blood through the leukapheresis and activates the T-cell through Anti-CD3 / Anti-CD28 Abcoated beads.
Afterward, CAR-T is created after CAR gene expression using the gene transfer, and the CAR gene is transduced into activated T-cell. Before infusing it back into the patient, the number of CAR-T cells is increased through culture step, harvests and refrigerates them. [5]
Our New Innovative product, ADAMⅡ-CDx (coming soon)
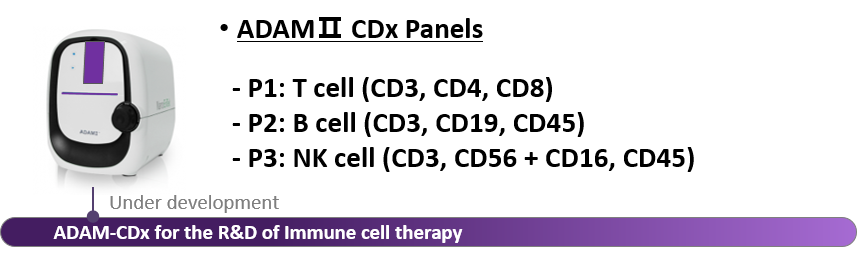
ADAM-CDx from NanoEntek is currently under development. This device can be used as an all-in-one in the cell therapy's manufacturing process and R&D from leukapheresis to product QC.
ADAM-CDx is specialized for various cells such as the T cell, B cell and NK cell to identify types of cell and utilized various functions such as total cell counting, cell size and cell cycling.
Reference paper of using ADAM-MC2
[3] Heterozygous frameshift variants in HNRNPA2B1 cause early-onset oculopharyngeal muscular dystrophy, Nature Communications, 13, 2306, 2022
Reference
[1] Future of Cell Therapy in the Regenerative Medicine Market(2016), Frost&Sullivan, 나노엔텍 재가공
[2] Viardot, Andreas, et al. "Chimeric antigen receptor(CAR) T-cell therapy as a treatment option for patients with B-cell lymphomas: perspectives on the therapeutic potential of Axicabtagene ciloleucel." Cancer management and research 11(2019): 2393
[3] CHAN, J. K. C., NG, C. S., HUI, P. K.(1988). “A simple guide to the terminology and application of leucocyte monoclonal antibodies”. 《Histopathology》 12(5): 461–480.
[4] Huh, D.; WeiGu; Kamotani, Y.; Grotberg, J. B.; Takayama, S.Physiologicla Measurement 2005, 26, R73-R98.
[5] 채희정, and 윤덕현. "광범위큰 B 세포림프종에 대한 키메릭항원수용체 T-세포(Chimeric Antigen Receptor T-cells) 치료." Korean Journal of Medicine(구 대한내과학회지) 94.2(2019): 152-158.
- ADAM-CellT_Performance Evaluation of CAR-T cell KAI.pdf
- ADAM-MC2_Application note_Mammalian cell.pdf
- ADAM-MC2_ Polymorphic Region-Specific Antibody for Evaluation of Affinity-Associated Profile of Chimeric Antigen Receptor.pdf
- TDP-43 and PINK1 mediate CHCHD10S59L mutation_induced defects in Drosoph....pdf

 KOR
KOR ENG
ENG